By Jeff Poland
An analysis of data collected from patients in cardiac arrest, which was published in the British Medical Journal, discussed the timing of administration of epinephrine in in-hospital cardiac arrests. Specifically, the authors wanted to know if the timing of the administration of epinephrine in initial shockable rhythms affected patient-important outcomes — defined in the study as survival to hospital discharge absent neurologic sequelae — in patients who experienced an in-hospital cardiac arrest.
Anderson et al conducted their study in a retrospective fashion, which is looking at past data from a registry that was not collected specifically for the purpose of this study, using the Get With The Guidelines – Resuscitation registry, which collects information from over 300 hospitals in the U.S. with regard to how closely resuscitations are ran as compared to the current AHA guidelines.
The registry collects data in a prospective, which means the data points were determined prior to the start of the registry, manner for the purpose of seeing how closely the AHA guidelines are adhered to. The authors only included data from patients who presented in ventricular tachycardia or ventricular fibrillation, and received their initial defibrillation within two minutes and then remained in a shockable rhythm.
They excluded a fairly large number of patients including those who received epinephrine before the first defibrillation, who achieved ROSC before two minutes post first defibrillation, those who were not admitted to the hospital and those with incomplete data sets, among others. This is important when you’re looking at such a specific outcome with data that wasn’t tailor-made for the study.
The authors looked at the data, using statistics to control for confounders, which are other factors that may influence the data in one way or another, using a statistical technique called 1:1 propensity matching. Propensity matching entails finding patients with similar clinical characteristics, such as age and comorbidities, but who had different interventions performed, and then placing one of each into each cohort or group of the trial.
For example, patient A, a 56-year-old female who was admitted for renal failure with three comorbid conditions, presented in ventricular tachycardia and received epinephrine at one minute post defibrillation. Patient B, a 54-year-old female who was admitted for renal failure with three comorbid conditions, presented in ventricular tachycardia and did not receive epinephrine. Patient A would be placed into the “less than two minute” cohort and patient B into the “no epinephrine within first two minutes” cohort. Patients A and B are then compared 1:1.
From 310 hospitals, a total of 2,978 patients were matched into either the “epinephrine within two minutes” cohort or the “no epinephrine within first two minutes” cohort. Epinephrine was strongly associated with decreased odds of ROSC, survival and good neurologic outcome on discharge.
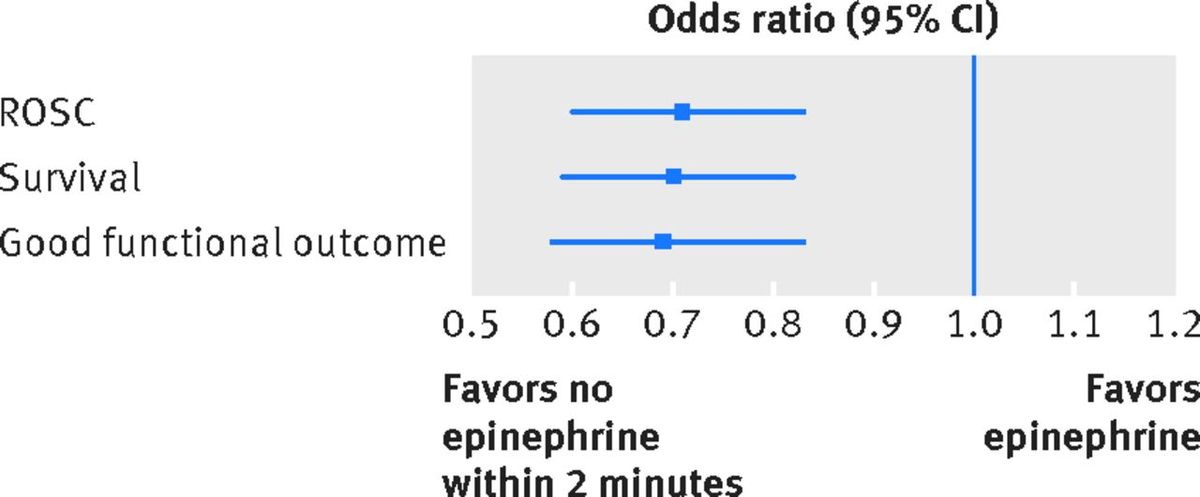
Odds ratio with 95 percent Confidence Intervals showing harm associated with early epinephrine use in shockable rhythm. Anderson et al, BMJ 2016, figure 4.
An odds ratio is used to show the odds of harm or benefit from an intervention. The further away from 1.0 the higher the probability that the intervention has benefit (greater than 1.0) or has harm (less than 1.0). The bars in the chart show the confidence interval, a statistical range in which the true effect has a 95 percent chance of falling. In this case, pretty far into the harm category.
Memorable quotes on early epinephrine use in initial shockable rhythm
Anderson et al make a number of good points in their research, but these three stand out.
"… [A]dministration of epinephrine within the first two minutes after the initial defibrillation was common and was associated with a decreased chance of return of spontaneous circulation, survival, and survival with good functional outcome.”
"… [W]e found that the proportion of patients who received early epinephrine has been increasing over the years (from 46 percent in 2006 to 60 percent in 2012), a finding that remains largely unexplained.”
“Epinephrine’s beta-adrenergic effects could increase myocardial oxygen demand, which, despite increased coronary perfusion pressure, can lead to increased myocardial damage and increased myocardial dysfunction after cardiac arrest. Epinephrine can also decrease blood flow to other organs including the kidneys, lungs and brain, as well as microcirculatory blood flow.”
Key takeaways: Epinephrine in shockable rhythms
While one study, especially not a retrospective cohort analysis, should change one’s practice, here are five things that are common to all research into epinephrine administered during cardiac arrest resuscitation.
1. The effects of epinephrine are unproven
Epinephrine was adopted into clinical guidelines in the 1970s based upon a small scale study of a Beagle model of cardiac arrest. It consistently shows a dramatic improvement in ROSC, but not in patient-important outcomes like neurologic function or survival to hospital discharge, and in most studies epinephrine administration has even shown harm [2,3,4,5,6,7,8,9].
2. ACLS algorithms are not definitive treatment
While the ACLS and BLS guidelines provide a consensus model for how to provide circulatory support in cardiac arrest, the real focus shouldn’t be on how much epinephrine you can push during an arrest. It should be focused on identifying and correcting the cause of arrest. Remember for most adults, the underlying cause is often cardiac-ischemic and epinephrine might not be helpful.
3. In-hospital arrests are different from out of hospital arrests
While this research focused on in-hospital arrests, out-of-hospital arrests are different in that the time to defibrillation and drug administration is significantly increased. Universally, delayed administration of epinephrine is associated with poor outcomes, and the current AHA guidelines even recommend early administration of epinephrine, recognizing the poor outcomes [10]. It is therefore crucial to consider the timing of epinephrine administration and consider forgoing it entirely.
4. Focus on quality chest compressions
Consistently, the only intervention which has been shown to improve outcomes is high-quality chest compressions [10]. If your agency doesn’t already use a pit crew model of arrest, you’re still pausing compressions to intubate or you’re not receiving feedback on measurements such as compression fraction, you should strongly consider changing your practice and protocols.
5. Bystander CPR is crucial
In out-of-hospital cardiac arrests, it can be five to 10 minutes before EMS arrives. Bystander CPR and access to defibrillation is crucial in good outcomes. What does your agency do to promote bystander CPR training?
What are your top takeaways from research on the administration of epinephrine during cardiac arrest resuscitation?
About the author
Jeff Poland, NRP, FP-C is a critical care paramedic in Washington state with a passion for critical care and pharmacology. A self-professed critical care nerd, Jeff avidly follows all of the podcasts that end in "-crit” and strives to ride the bell curve between innovation and early adoption to deliver the best care to his patients. He welcomes any and all correspondence at jeff.poland@gmail.com
References
1. Anderson L, Kurth T, Chase M, et al. Early administration of epinephrine (adrenaline) in patients with cardiac arrest with initial shockable rhythm in hospital: propensity score matched analysis. BMJ2016;353:i1577. doi:10.1136/bmj.i1577.
2. Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med2009;37:1408-15. doi:10.1097/CCM.0b013e31819cedc9 pmid:19242339.
3. Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation2011;82:1138-43.doi:10.1016/j.resuscitation.2011.06.029 pmid:21745533.
4. Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA2009;302:2222-9. doi:10.1001/jama.2009.1729 pmid:19934423.
5. Dumas F, Bougouin W, Geri G, et al. Is epinephrine during cardiac arrest associated with worse outcomes in resuscitated patients?J Am Coll Cardiol2014;64:2360-7. doi:10.1016/j.jacc.2014.09.036 pmid:25465423.
6. Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation1995;92:3089-93. doi:10.1161/01.CIR.92.10.3089 pmid:7586280.
7. Fries M, Weil MH, Chang YT, Castillo C, Tang W. Microcirculation during cardiac arrest and resuscitation. Crit Care Med2006;34(Suppl):S454-7. doi:10.1097/01.CCM.0000247717.81480.B2 pmid:17114977.
8. Behringer W, Kittler H, Sterz F, et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann Intern Med1998;129:450-6. doi:10.7326/0003-4819-129-6-199809150-00004 pmid:9735082.
9. Arrich J, Sterz F, Herkner H, Testori C, Behringer W. Total epinephrine dose during asystole and pulseless electrical activity cardiac arrests is associated with unfavourable functional outcome and increased in-hospital mortality.Resuscitation2012;83:333-7. doi:10.1016/j.resuscitation.2011.10.027 pmid:22079948.
10. Link M, Berkow L, Kudenchuk P, et al. Adult Advanced Cardiovascular Life Support. Circulation 2015; 132: S444-S464. doi: 10.1161/CIR.0000000000000261.











