Highlights
Introduction
Engine 19 and Rescue 9 respond to a report of an unconscious person. The engine arrives first on the scene and reports that cardiopulmonary resuscitation (CPR) is in progress.
The rescue arrives to find a male, age 68, lying on the floor with two firefighters performing high-quality CPR. The automated external defibrillator (AED) applied by the engine crew delivered a single shock about 45 seconds before the paramedics enter the house.
Without an interruption in chest compressions, Paramedic Avalos quickly inserts a supraglottic airway while Paramedic Sandoval gains intravenous access. Before Sandoval can administer epinephrine, the Code Commander announces that the two-minute CPR interval will end in 15 seconds.
At the two-minute mark, the AED analyzes the rhythm and recommends a shock. Avalos delivers the shock without incident, the two firefighters switch positions, and they resume CPR. Avalos plugs the defibrillator cable into the manual monitor defibrillator and discovers an organized rhythm. Avalos also notices an abrupt increase in the end-tidal carbon dioxide value but instructs the firefighters to complete the two-minute cycle of CPR. Sandoval decides not to administer the epinephrine.
At the next pulse check, Avalos detects a strong carotid pulse. The electrocardiogram (ECG) displays a sinus tachycardia. The patient makes no attempt to breathe on his own and Sandoval begins assisting the patient’s ventilation. The patient’s blood pressure is 98/66 mm Hg and the pulse rate is 110 beats per minute.
Before placing the patient onto a backboard, Avalos indicates that he wants to obtain a 12-lead ECG. The results suggest an evolving myocardial infarction. During transport, Avalos informs the receiving hospital that the patient meets the ECG criteria for cath lab activation.
When the rescue arrives at the hospital, the staff directs the medics to place the patient in an emergency department (ED) room where they will repeat the ECG before alerting the cath lab. The doctor explains that first ECG tracings can sometimes be misleading following successful out-of-hospital resuscitation.
Review
For patients presenting with signs and symptoms of acute ischemic chest pain, the American Heart Association (AHA) places the 12-lead ECG at the center of the clinical decision making pathway (Sinz, Navarro, & Soderberg, 2011).
For patients who suffer cardiac arrest, the AHA directs healthcare providers to obtain a 12-lead ECG as soon as possible after achieving return of spontaneous circulation (ROSC) to identify those patients with ST-segment myocardial infarction or new or presumably new left bundle branch block (Peberdy et al., 2010).
Early acquisition of the 12-lead ECG allows healthcare providers to quickly identify those patients likely to receive the greatest benefit from coronary reperfusion interventions (O’Connor et al., 2010).
However, healthcare providers may have difficulty interpreting out-of-hospital ECG changes following ROSC (Dumas et al., 2010; Spaulding et al., 1997) as acute ischemia-reperfusion syndrome in these patients may produce significant ECG changes even in the absence of acute myocardial infarction (Adrie et al., 2002; Edgren, Kelsey, Sutton, & Safar, 1989).
In France, all victims of cardiac arrest who achieve ROSC routinely receive emergency coronary angiography (ECA) and induced hypothermia as soon as they arrive at the hospital.
Coronary angiography is the most definitive intervention for diagnosing coronary artery blockage. If the angiogram reveals blockage, cardiologists perform immediate angioplasty. In the absence of arterial blockage, physicians transfer the patient to an intensive care unit for standard post-resuscitation care.
In this study, researchers wanted to know if a post-resuscitation 12-lead ECG could accurately predict who actually needs ECA and who does not (Sideris et al., 2011).
This study was a retrospective chart review of all patients over the age of 18 years who suffered an out-of-hospital cardiac arrest and arrived at a single receiving facility during a 66-month period. The review team excluded patients with non-cardiac causes of cardiac arrest, patients who did not sustain ROSC for at least 20 minutes, and those without an available 12-lead ECG following ROSC.
The researchers used the first interpretable 12-lead ECG obtained after the patient achieved ROSC. The research team blinded all ECG reviewers to the results of the angiogram, meaning the reviewers did not know who actually had coronary artery blockage and who did not.
Two experienced physicians interpreted each ECG and a third party resolved any disagreement between the two reviewers. Specifically, the reviewers looked for any ECG changes predictive of the need for emergency angiography, such as ST-segment elevation (STE), ST-segment depression (STD), the presence of either left or right bundle branch block (BBB), or non-specific wide QRS complex (WQRS).
Two experienced and independent reviewers also examined the results of each angiogram and a third party resolved any disagreements. The reviewers assessed blood flow through the coronary arteries using the Thrombolysis in Myocardial Infarction (TIMI) classification, defined acute myocardial infarction by the European Society of Cardiology criteria, and excluded cases of chronic coronary artery occlusion.
Results
Researchers enrolled 165 patients into the study. Most were male with an average age of 56 years. Some type of acute coronary event was the cause of the cardiac arrest in about 70 percent of the cases with acute myocardial infarction (AMI) responsible for slightly over one-third (36 percent) of the arrests. A complete description of the causes of cardiac arrest in this patient sample appears in Chart 1.
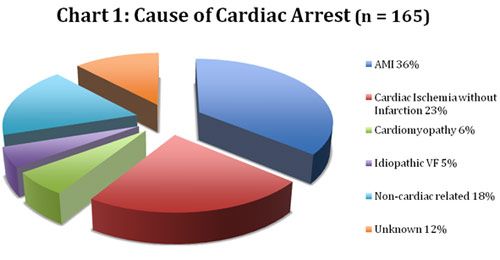
|
Only 60 (36 percent) of the 165 patients had angiographic evidence of AMI, meaning that 105 patients received ECS who did not need the procedure. Physicians (and presumably paramedics) using the presence of STE as the sole determining criterion of the need for ECA would have identified about 88 percent of all the patients who really did have an AMI. That same criterion would have resulted in 17 patients receiving an angiogram who did not need one (16 percent false positive rate).
While this is impressive, cardiologists using that criterion would not have performed emergency angiography or angioplasty on 7 patients (12 percent) who actually did have coronary artery blockage. One might argue that any test that misses 12 percent of patients who actually do have a problem is unacceptably high.
To decrease this number, emergency personnel would need to expand the criteria beyond STE to include other potential indicators of infarction. This process is known as increasing sensitivity of the diagnostic test.
Although desirable, increasing sensitivity comes with a cost. Including additional ECG changes as indicators of the need for angiography will decrease the chances of missing someone who actually is having an AMI, but will also result in performing angiography in more patients who actually do not have an AMI (false positives, also known as specificity).
By using both STE and STD as indicators of the need for angiography, the emergency team would have identified 95 percent of the patients who actually had an AMI (95 percent sensitivity), but would also have resulted in an unnecessary angiogram for 39 patients (62 percent specificity). Three patients who actually had an AMI would not have received an angiogram, which some might argue is still too high, especially if you are one of the three.
Adding the presence of left bundle branch block (LBBB) and WQRS to STE and STD would have identified all of the patients who actually had an AMI (100 percent sensitivity) and resulted in 49 patients receiving an angiogram who did not need one (46 percent specificity). Adding right bundle branch block (RBBB) to the criteria would not have changed the specificity, which was already at 100 percent, but would have resulted in 74 patients receiving an angiogram who did not need one (39 percent specificity).

|
What This Means For You
This is the first study to use a comprehensive list of 12-lead ECG changes to determine who would benefit from ECA following successful resuscitation from out-of-hospital cardiac arrest. Two previous investigations suggest improved survival following out-of-hospital cardiac arrest due to AMI if physicians perform coronary angioplasty during the post resuscitation period (Dumas et al., 2010; Spaulding et al., 1997).
However, the availability of ECA is limited, the procedure is expensive, and does not benefit all patients who suffer an out-of-hospital cardiac arrest (Garot et al., 2007; Gorjup, Radsel, Kocjancic, Erzen, & Noc, 2007).
This study suggests that prehospital personnel can accurately use the first 12-lead ECG following ROSC to determine who needs ECA, thus eliminating the costs associated with universal angiography for patients who achieve ROSC. However, 12 percent of the patients in the study who actually had coronary artery occlusion did not have STE on the first 12-lead ECG following ROSC.
To reduce the chances of withholding or delaying angioplasty for patients who need it, prehospital personnel may need more inclusive criteria for ECG changes suggestive of acute ischemia or infarction beyond STE, such as STD, the presence of left or right bundle branch block, and/or WQRS.
Limitations
This investigation was a retrospective review of patient charts. Since retrospective reviews look at events that already happened, the researchers cannot control variables that might affect the research question or hypothesis. Retrospective reviews are useful for gaining insight into an issue, but they cannot provide definitive answers to research questions.
Twelve percent of the patients who actually had some degree of coronary artery blockage did not have STE on the first 12-lead ECG following ROSC. The authors do not make it clear whether the emergency department team initiated therapeutic hypothermia in these patients before obtaining the ECG.
It is possible that cooling may have blunted the ECG changes (Tan & Meregalli, 2007) thereby reducing the sensitivity of the initial 12-lead ECG in indentifying the need for ECA.
Advanced life support ambulances in France are staffed by physicians and therefore, one cannot easily generalize on-scene resuscitation techniques in that country to American prehospital resuscitation scenes.
Some might use this difference to discount the results of this study; their argument being the prehospital providers in the two countries are not equal; therefore one cannot take something that works in France and apply it in the United States.
However, this study is not evaluating the quality of the resuscitation attempt, instead evaluating whether a 12-lead ECG obtained after cardiac arrest can predict the need for angioplasty. As such, the education and training of the prehospital provider is not a limitation to the generalizability of the results.
However, this study only included patients brought to a single teaching hospital and not all patients who suffered an out-of-hospital cardiac arrest. Based on the information contained within the paper, the study sample represented only about 16 percent of all of the patients in the city who were admitted alive to hospitals after suffering an out-of-hospital cardiac arrest.
The authors admit that because of the ECA services available at the study facility, the field crews may have selected this subgroup of patients for transport to this specific location. The authors provide no data comparing the characteristics of the study patients to the group of patients transported to a different location or to the total population of patients in the city who suffered an out-of hospital cardiac arrest.
One cannot be sure that using the same diagnostic criteria in a different city, or even in a different part of the same city would produce similar results. Although the 12-lead ECG identified all of the patients with true coronary artery blockage in this study, other healthcare providers with different subgroups of patients may need 15-lead or right side 12-lead ECGs to identify all of the true infarctions.
In conclusion, EMS providers should perform a 12-lead ECG as soon as possible after achieving ROSC following out-of-hospital cardiac arrest. If the patient demonstrates ECG evidence of infarction, medics should transport patients to a facility best equipped to perform emergency coronary revascularization techniques.
EMS agencies and their medical directors should work closely with their local receiving facilities to determine the optimal ECG diagnostic criteria for those procedures.
References
Adrie, C., Adib-Conquy, M., Laurent, I., Monchi, M., Vinsonneau, C., Fitting, C., Fraisse, F., Dinh-Xuan, A. T., Carli, P., Spaulding, C., Dhainaut, J. F., & Cavaillon, J. M. (2002). Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation, 106(5), 562–568.doi: 10.1161/01.CIR.0000023891.80661.AD
Dumas, F., Cariou, A., Manzo-Silberman, S., Grimaldi, D., Vivien, B., Rosencher, J., Empana, J. P., Carli, P., Mira, J. P., Jouven, X., & Spaulding, C. (2010). Immediate percutaneous coronary intervention is associated with better survival after out-of hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circulation: Cardiovascular Interventions, 3(3), 200–207. doi:10.1161/CIRCINTERVENTIONS.109.913665
Edgren, E., Kelsey, S., Sutton, K., & Safar, P. (1989). The presenting ECG pattern in survivors of cardiac arrest and its relation to the subsequent long-term survival. Brain Resuscitation Clinical Trial I Study Group. Acta Anaesthesiologica Scandinavica, 33(4), 265–271.
Garot, P., Lefevre, T., Eltchaninoff, H., Morice, M. C., Tamion, F., Abry, B., Lesault, P. F., Le Tarnec, J. Y., Pouges, C., Margenet, A., Monchi, M., Laurent, I., Dumas, P., Garot, J., & Louvard Y. (2007). Six-month outcome of emergency percutaneous coronary intervention in resuscitated patients after cardiac arrest complicating ST-elevation myocardial infarction. Circulation, 115(11), 1354–1362. doi: 10.1161/CIRCULATIONAHA.106.657619
Gorjup, V., Radsel, P., Kocjancic, S. T., Erzen, D., & Noc, M. (2007). Acute ST-elevation myocardial infarction after successful cardiopulmonary resuscitation. Resuscitation, 72(3), 379–385. doi:10.1016/j.resuscitation.2006.07.013
O’Connor, R. E., Brady, W., Brooks, S. C., Diercks, D., Egan, J., Ghaemmaghami, C., Menon, V., O’Neil, B. J., Travers, A. H., & Yannopoulos, D. (2010). Part 10: Acute coronary syndromes: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation, 122(suppl 3), S787–S817. doi:10.1161/CIRCULATIONAHA.110.971028
Peberdy, M. A., Callaway, C. W., Neumar, R. W., Geocadin, R. G., Zimmerman, J. L., Donnino, M., Gabrielli, A., Silvers, S. M., Zaritsky, A. L., Merchant, R., Vanden Hoek, T. L., & Kronick, S. L. (2010). Part 9: Post– cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation, 122(suppl 3), S768 –S786. doi:10.1161/CIRCULATIONAHA.110.971002
Sideris, G., Voicu, S., Dillinger, J. G., Stratiev, V., Logeart, D., Broche, C., Vivien, B., Brun, P., Deye, N., Capan, D., Aout, M., Megarbane, B., Baud, F. J., & Henry, P. (2011). Value of post-resuscitation electrocardiogram in the diagnosis of acute myocardial infarction in out-of-hospital cardiac arrest patients. Resuscitation, 82(9), 1148-1153. doi:10.1016/j.resuscitation.2011.04.023
Sinz, E., Navarro, K., & Soderberg, E. S. (2011). Advanced cardiovascular life support. Dallas, TX: American Heart Association.
Spaulding, C. M., Joly, L. M., Rosenberg, A., Monchi, M., Weber, S. N., Dhainaut, J. F., & Carli, P. (1997). Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. New England Journal of Medicine, 336(23), 1629–1633.
Tan, H. L., & Meregalli, P. G., (2007). Lethal ECG changes hidden by therapeutic hypothermia. Lancet, 369(9555), 78. doi.org/10.1016/S0140-6736(07)60034-8












