By Ben Dowdy
One of the questions I struggle with regularly is one of when to switch from replacing fluid to starting vasopressors. It was easier when I was in paramedic school; you almost never needed to. “Leave ‘em dead with Levophed!” was our battle cry, and the scenarios for cardiogenic shock were picture-perfect and always responded to dopamine.
Then I took care of some really sick patients in the real world, and things got trickier.
The physiology behind choosing between adding IV fluids to the patient’s cardiovascular system and stressing what’s already in there with vasopressors seems straightforward; the Frank-Starling curve. On the left-hand side of the curve are patients whose left ventricle isn’t optimally tensioned by preload yet, and adding fluids will benefit the patient by “stretching” the myocardial fibers, increasing stroke volume, and improving cardiac output. On the right-hand side of the curve are patients who have adequate preload already; they won’t benefit (and might be harmed) by additional IV fluids, and stressing the volume that’s already hanging out in their venous system is the logical choice to make.
Unfortunately for us, every patient starts at a different point along the Frank-Starling curve, depending on the severity and etiology of their hypoperfusion. Additionally, the really sick patients we take care of often have varying degrees of cardiac disease or dysfunction, which alters the shape of their personal Frank-Starling curve. For these reasons, deciding when enough is enough with the IV boluses is a lot more challenging than you might think. Critical care physicians have been aware of this for some time, and have been coming up with a few techniques to help make this decision:
1. Ultrasound view of the inferior vena cava
Due to the mechanics of breathing, the inferior vena cava (IVC) often collapses a bit whenever we breathe in; ordinarily, this is no big deal. However, in patients who need some additional fluid, the IVC collapses more than normal during inspiration. This has been termed the “IVC collapsibility index;” by viewing the IVC with ultrasound and measuring the degree to which it collapses during inhalation and exhalation, you can predict who will respond to additional fluid and who needs vasopressor support.
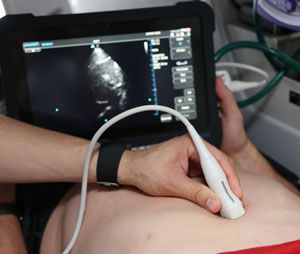 |
| The IVC collapsibility index predicts which patients will respond to additional fluid or if vasopressor support is needed. (Photos by: Jason Clark, LIFE FORCE Clinical Educator) |
2. Pulse pressure variation
You may remember that pulse pressure is the difference between systolic and diastolic blood pressures; you may even remember it has something to do with cardiac tamponade and Beck’s Triad. Since systolic blood pressure is primarily affected by stroke volume coming out of the left ventricle (which should be increased by increasing preload), the pulse pressure can be used as an indicator for fluid responsiveness.
In the ICU, physicians calculate the pulse pressure variation by looking at an invasive arterial blood pressure waveform after administering a fluid bolus; if the pulse pressure significantly increases after the bolus, then the patient needs more fluid.
3. Stroke volume variation
Of course, if you’re in an ICU and have an arterial line in place, you might also have a pulmonary artery catheter capable of doing LOTS of neat things in place as well! Some have suggested that directly measuring the stroke volume before and after a fluid bolus can help to determine if a patient needs additional boluses; if the stroke volume doesn’t increase significantly, no need for further boluses.
4. Passive leg raising
The downside to using pulse pressure or stroke volume variation as a test of fluid responsiveness is that you need to give a fluid bolus (not standardized, but typically around 500mL); if the patient doesn’t respond, you’ve just given them half-a-liter of fluid they don’t need. Passive leg raising uses a physiological “fluid bolus” from the venous reserve in the legs in place of exogenous fluid. By placing the patient first in the semi-Fowler’s position, then a Trendelenburg position, a bolus of blood reaches the central circulation and its effect on pulse pressure or stroke volume variation can be measured.
There are a few other techniques as well, and all of them have differing degrees of accuracy and limitations. Read a great review on Hemodynamic parameters to guide fluid therapry.
All this seems fascinating, but what good does it do me if I don’t have an ultrasound, an invasive arterial line, or a pulmonary artery catheter?
For all the fluid we can push on our critically ill and injured patients and the vaso-active medications in our various drug boxes and bags, we don’t have a reliable way of deciding when enough fluid is enough. Blood pressure, or even mean arterial pressure, just isn’t specific enough to stroke volume for us to make good choices in this regard. There are a couple of less-than-ideal options for those of us without invasive hemodynamic monitoring, however:
- The pleth waveform of your pulse oximeter can be used in a similar manner to IVC collapsibility; if the size of the waveform decreases significantly during inhalation, a fluid bolus might be indicated.
- A relatively small study of mechanically ventilated patients found that an increase of end-tidal carbon dioxide >5% after passive leg raising correlated well with an increase in stroke volume (and therefore that the patient would respond to fluids).[1]
However, I think a better long-term solution would be to incorporate non-invasive hemodynamic monitoring into future multi-parameter cardiac monitors .
Non-invasive technology now exists to measure hemodynamics that were only available with arterial lines and Swan-Ganz catheters; why not make use of them?
Regardless of what monitoring technology you have available, and what methods you use to check fluid responsiveness, knowing exactly when your patient has had just enough fluid continues to be one of the more challenging goals in resuscitation.
References
1. Monnet X, Bataille A, Magalhaes E, et al. “End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test.” Intensive Care Med 2013 Jan;39(1):93-100.
About the Author
Ben Dowdy B.S., NR-P currently works in northern Idaho for a rural/frontier EMS system. His past experiences include work as a field provider, tactical paramedic, paramedic for the National Parks Service, and EMS educator. He is a lead instructor for Wilderness Medical Associates, for whom he has taught medical courses for providers of all levels throughout the US as well as abroad.











