By Kelly Grayson
Traumatic brain injury, hemorrhagic stroke and spinal cord injury are three pathologies that can result in devastating neurological injury. While we can do little in the prehospital environment to treat them, careful assessment of the patient, including waveform capnography, can alert us to potential life-threatening sequelae and help us make sure our interventions do not inadvertently make the patient worse.
Traumatic brain injury accounts for nearly 40 percent of all trauma deaths in the United States, approximately 52,000 deaths per year. Roughly 200,000 TBI victims yearly require hospitalization, at a cost of nearly $4 billion per year in medical expenses, lost wages and productivity. The mortality rate for severe TBI — Glasgow Coma Score < 8 — is roughly 33 percent [1].
Hemorrhagic stroke accounts for nearly 13 percent of the 795,000 strokes yearly in the United States, of which 3 percent are subarachnoid hemorrhage and 10 percent are intracerebral hemorrhage [2, 3]. Prognosis in these patients is based upon location, size and severity of hemorrhage as defined by Glasgow Coma Scale.
The presence of a large volume of blood at presentation and growth of hematoma size are associated with higher mortality. The Intracerebral Hemorrhage score is the most commonly used prognostic indicator for hemorrhagic stroke, and is calculated thusly:
| Intracerebral Hemorrhage Assessment | Points |
| GCS score 3-4 | 2 |
| GCS score 5-12: | 1 |
| GCS score 13-15 | 0 |
| Age ≥ 80 years | Yes=1 No=0 |
| Infratentorial origin: | Yes=1 No=0 |
| Intracerebral hemorrhage volume ≥30 cm3 | 1 |
| Intracerebral hemorrhage volume < 30 cm3 | 0 |
| Intraventricular hemorrhage | 0 |
Each increase in the patient’s ICH score is associated with an increase in 30-day mortality; those patients with an ICH score of 3 had 72 percent mortality, those scoring 4 had 97 percent mortality. All patients with an ICH score of 5 died [4].
Approximately 12,500 people in the United States suffer spinal cord injuries every year, and it is estimated that there are as many as 276,000 SCI survivors in the United States as of 2014 [5]. Patients with incomplete and complete tetraplegia represent 30.1 percent and 20.4 percent of SCI victims, respectively. These two categories represent over half of SCI patients, and are at highest risk of respiratory dysfunction.
While these three pathologies — traumatic brain injury, spinal cord injury and hemorrhagic stroke — may represent a broad constellation of clinical syndromes ranging from minor to potentially devastating neurological sequelae, we will limit our focus to the most severe of each, which may pose high risk of severe respiratory and vascular dysfunction. Proper care of these patients can be guided with the use of quantitative waveform capnography.
Primary and secondary neurological injury
Injury resulting from severe neurological trauma can be classified into two broad categories: primary and secondary.
Primary injury occurs at the time of and in the area of the original insult, while secondary injury may occur hours to days or weeks afterward the initial insult. While little can be done in the prehospital realm to limit or reverse primary injury, careful assessment and monitoring, including quantitative waveform capnography, can provide clues to the caregiver of the impending neurological sequelae of secondary injury.
Although hemorrhagic stroke — or other causes of intracranial bleeding — has not been typically classified into primary and secondary syndromes, one might consider the mass effect of an expanding intracranial hemorrhage to be a secondary injury.
Traumatic brain injury and hemorrhagic stroke
Primary injury in TBI can be classified into two broad categories: focal and diffuse.
Focal injuries affect one discrete part of the brain and include skull fractures, penetrating wounds, intracranial hematomas and the like, while diffuse injuries are more widespread, such as diffuse axonal injury.
Diffuse axonal injury is present in nearly half of severe TBI and its primary symptom is prolonged unconsciousness. DAI may be difficult to identify with neuroimaging, as most of the changes are microscopic.
While focal TBI is primarily caused by direct impact, DAI is caused by indirect force, typically the acceleration-deceleration phenomenon common in automobile accidents, sports impacts, and blast injuries. Cerebral axons bridging different brain regions are stretched and damaged by rapid acceleration or deceleration.
While it was long thought that the axonal tracts are sheared as a result of primary injury, we now know that these axons are merely damaged, and the biochemical cascade that results in secondary injury is responsible for most of the neuron death. Excitatory amino acids such as glutamate and aspartate are released in large quantities after primary injury, and these biochemical transmitters are largely responsible for cellular edema.
The axonal tracts commonly damaged in DAI are concentrated in the corpus callosum, cerebral hemispheres and the brain stem. It is the damage to the brain stem that is most likely to cause severe derangements in vascular and respiratory function.
Monro-Kellie doctrine
Whether the insult to brain tissue results from focal impact, diffuse axonal injury, intracranial hemorrhage or the mass effect resulting from it, the syndrome we wish to avoid is increased intracranial pressure.
The Monro-Kellie doctrine holds that the cranial vault is a non-expandable chamber, and as a result, the volume of that chamber remains constant. Consequently, the three elements occupying that chamber — blood, cerebrospinal fluid and brain tissue — must remain in constant equilibrium.
If the volume of one of the elements increases, the volume of one or both of the other elements must correspondingly decrease to maintain that equilibrium. An increase in intracranial pressure from cerebral edema or an expanding hematoma can compress and damage surrounding brain tissue, impair cerebral blood flow, cause brainstem herniation and result in profound neurological damage or death.
Normally, cerebral perfusion pressure remains constant due to autoregulation and may vary from 70-85 mm Hg. For patients with abnormal mean arterial pressure or abnormal intracranial pressure, the cerebral perfusion pressure is calculated by subtracting the intracranial pressure from the mean arterial pressure:
CPP = MAP − ICP
One of the main dangers of increased ICP is that it can cause ischemia by decreasing CPP. As ICP approaches mean arterial pressure, cerebral perfusion falls. The body responds to this fall in CPP by raising blood pressure and dilating cerebral blood vessels. This increases cerebral blood volume, further worsening ICP and starting a vicious cycle that lowers CPP even further.
Recognition of low-perfusion states with capnography
Quantitative waveform capnography can help us recognize hypoperfusion states early — remember, MAP is essential in maintaining CPP — and recognizing the signs of increased ICP. While volume resuscitation does not significantly increase CPP in patients with intact autoregulation, patients with TBI may have severely impaired autoregulation. A number of studies have demonstrated that hypotension — systolic blood pressure < 90 mm Hg — is one of the biggest predictors of mortality and the generally accepted recommendation is to maintain a physiologically normal MAP of 65 mm Hg [6,7,8].
Since quantitative waveform capnography is an excellent indirect measure of perfusion, the astute clinician may be alert to falling ETCO2 levels as a precursor to frank hypotension and titrate fluid therapy accordingly.
While an ETCO2 of < 25 mm Hg was strongly associated with serum lactate > 4 mmol/L in sepsis patients [9], the relationship between serum lactate and cerebral perfusion in TBI has not been firmly established.
Rather than focus on a specific ETCO2 threshold at which to initiate fluid therapy, simply correlate trends in ETCO2 readings with mean arterial pressure. If the trend of both is downward, it may be reasonable to initiate judicious fluid boluses before the patient’s systolic blood pressure falls.
For instance, a patient with a systolic blood pressure of 100 mm Hg may have a MAP of only 50 mm Hg and a downward-trending ETCO2. This patient needs fluid boluses to maintain a MAP of ≥ 65 mmHg.
Avoidance of inappropriate hyperventilation
Where waveform capnography really shines is in avoiding inappropriate patient hyperventilation. While traditionally, induced hypocapnea at 25-28 mm Hg was considered a prophylactic therapy for traumatic brain injury, it also significantly limits cerebral blood flow and may worsen cerebral ischemia and is associated with worse outcomes [10].
In the TBI patient, cerebral vascular response to CO2 levels may be significantly increased, and thus hypocapnea is likely to significantly decrease cerebral blood flow regionally in the damaged area [11,12,13]. Therefore, hyperventilation, if done, must be carefully controlled, even in patients with increased ICP.
Ventilations should be titrated to maintain the patient at the lower limit of eucapnia, which means an optimal or normal level presence of arterial carbon dioxide which is 35 mm Hg, and quantitative waveform capnography is essential to accurate titrate volume and rate of ventilations.
Recognition of abnormal respiratory patterns
Abnormal respiratory patterns such as Biot’s respirations, Cheyne-Stokes respirations and central neurogenic hyperventilation are sometimes present in TBI patients, and may signify lesions to the brain stem or impending brain herniation. Biot’s and Cheyne-Stokes are similar in that the patient exhibits periodic breathing, but differ in that Biot’s respirations typically involve breaths at similar volumes alternating with periods of apnea, while Cheyne-Stokes respirations exhibit a crescendo-decrescendo pattern alternating with periods of apnea.
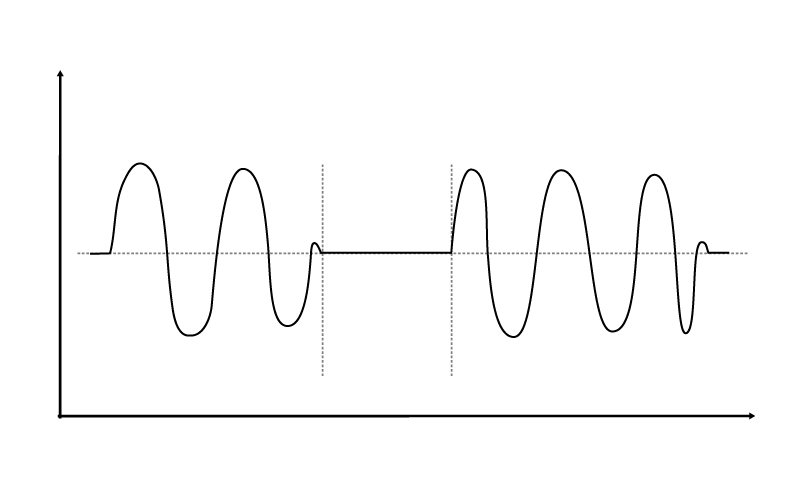
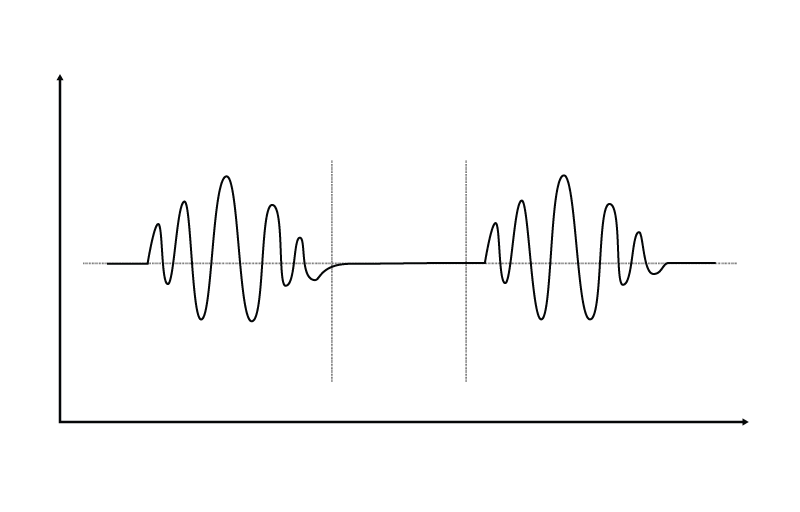
Central neurogenic hyperventilation occurs as a response to reduced carbon dioxide levels in the brain resulting from the constriction of cranial arteries associated with brainstem lesions.
While these patterns may not be readily apparent in the TBI patient who is being mechanically ventilated, they may be recognized in the spontaneously breathing patient. While your TBI or hemorrhagic stroke patient may not initially exhibit these abnormal respiratory patterns, they may occur as secondary injury begins to occur from edema.
A trend capnograph is useful in identifying these abnormal patterns.
Recognition of ventilatory dysfunction in patients with spinal cord injury
Patients with cervical spinal cord injury may exhibit some degree of respiratory dysfunction. Typically, patients with injury at the C1-C2 level retain only 5-10 percent of vital respiratory capacity and have no cough reflex, and those with C3-C6 injury only retain 20 percent of vital respiratory capacity, and cough reflex is weak and ineffective [14].
While a primary spinal cord lesion in these areas should be immediately obvious to caregivers, secondary injury from cord edema may be more insidious, particularly if the caregiver’s attention is directed elsewhere. Pay particular attention to rising ETCO2 levels indicative of hypoventilation, and provide ventilatory support as indicated. A trend capnogram may help providers recognize subtle changes in ventilatory efficiency over time.
Vascular dysfunction in patients with SCI
Patients with SCI above the level of T5-T6 may present with some degree of vascular dysfunction, resulting in hypotension, bradycardia, and hypothermia. While the effects of spinal shock are usually transient, true neurogenic shock must be managed with adequate temperature management and treatment of hypotension, either with judicious fluid boluses or pressors. Decreased ETCO2 levels, both from hypoperfusion and hypothermia, may alert you to impending neurogenic shock and allow timely intervention.
Summary
Severe traumatic brain injury, severe hemorrhagic stroke and high spinal cord injury all pose significant risk to vascular and respiratory function. Thorough assessment, including the use of quantitative waveform capnography, will help you identify lethal derangements in perfusion and ventilation, and guide your management of these dysfunctions.
References
1. Segun TD. Traumatic Brain Injury (TBI): Definition and Pathology. Emedicine, Medscape. September 2015.
2. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997; 349(9061):1269-76
3. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3. 125(1):e2-e220.
4. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001 Apr. 32(4):891-7.
5. National Spinal Cord Injury Statistical Center (NSCIS). Spinal Cord Injury Facts and Figures at a Glance. February 2015.
6. Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. Journal of Neurosurgery, Supplemental. November 1991 / Vol. 75 / No. 1s / Pages S59-S66.
7. Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochirurgica, Supplementum. 1993;59:121-5.
8. Trauma.org: Cerebral Perfusion Pressure
9. Hunter CL, Silvestri S, Dean M, Falk JL, Papa L. End-tidal carbon dioxide is associated with mortality and lactate in patients with suspected sepsis. American Journal of Emergency Medicine. 2013 Jan; 31(1):64-71.
10. Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized controlled trial. Journal of Neurosurgery. 1991;75:731-9.
11. Marion DW1, Firlik A, McLaughlin MR. Hyperventilation therapy for severe traumatic brain injury. New Horizons. 1995 Aug;3(3):439-47.
12. Ausina A, Báguena M, Nadal M, Manrique S, Ferrer A, Sahuquillo J, Garnacho A. Cerebral hemodynamic changes during sustained hypocapnia in severe head injury: can hyperventilation cause cerebral ischemia? Acta Neurochirurgica, Supplement. 1998;71:1-4.
13. Soustiel JF1, Mahamid E, Chistyakov A, Shik V, Benenson R, Zaaroor M. Comparison of moderate hyperventilation and mannitol for control of intracranial pressure control in patients with severe traumatic brain injury--a study of cerebral blood flow and metabolism. Acta Neurochirurgica. 2006 Aug;148(8):845-51.
14. Chin LS. Spinal Cord Injuries. Emedicine, Medscape. July 2015.













